Longwood Biopharmaceuticals initiates clinical phase II study of LW402, a next-generation JAK1 inhibitor
Recently, Longwood Biopharmaceuticals announced the initiation of a Phase II clinical study of LW402, a next-generation JAK1 inhibitor. The clinical phase II study will target several autoimmune diseases such as Atopic Dermatitis and Rheumatoid Arthritis.
The JAK (Janus Kinase) kinase family is a popular target in recent years, and a variety of autoimmune diseases such as rheumatoid arthritis, vitiligo, and atopic dermatitis are related to abnormal activation of the JAK-STAT pathway. JAK inhibitors can inhibit JAK kinase and block the JAK-STAT signaling pathway, which has great therapeutic potential.
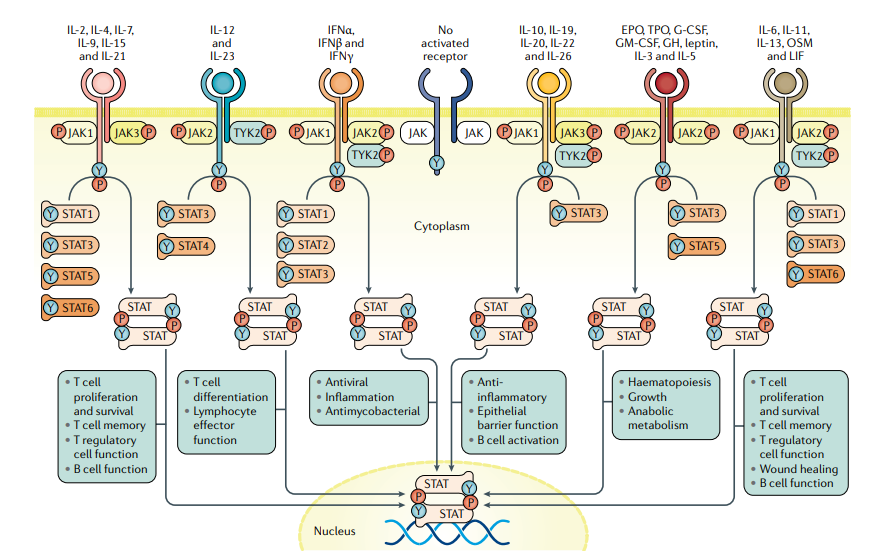
Since the first JAK inhibitor was launched in 2011, the JAK inhibitor market has featured a number of blockbuster drugs such as Ruxolitinib, Tofacitinib, and Upatinib, and the overall global market has now exceeded $9 billion. Despite the enthusiasm for research and development, selective JAK inhibitors have yet to break through safety challenges: JAK inhibitors produce off-target effects at higher doses, causing adverse reactions such as infections, venous thromboembolism (VTE), and dyslipidemia etc. The three marketed JAK inhibitors have black box warnings added to their instructions, and tofacitinib instructions are required by the FDA to add black box warnings for the risk of thrombosis and death. Upatinib and baricitinib have a black box warning for "risk of serious infection, malignancy, and thrombosis".
LW402 is a new generation JAK1 inhibitor developed independently by Longwood Biopharmaceuticals. Clinical phase I studies have shown excellent tolerability and safety, no significant dose-related AE, no target-related side effects, better average maximum inhibition of phosphorylation after multiple doses, no adverse effects such as infectious and thrombotic diseases were found, and is a potentially more effective drug.
Longwood Biopharmaceuticals will proceed rapidly with the multi-center clinical phase II study of LW402 and complete the commercialization process to bring LW402 to market as soon as possible. This will help Longwood Biopharmaceuticals to take its place in the field of autoimmune diseasez treatment and provide more treatment options to improve the quality of patients' lives.